Our team is committed to providing high-performance energy solutions tailored to your needs. To initiate further communication, kindly submit a formal inquiry for the product(s) of your interest.

Our team is committed to providing high-performance energy solutions tailored to your needs. To initiate further communication, kindly submit a formal inquiry for the product(s) of your interest.
If you’ve ever wondered why lithium-ion batteries are used in smartphones and EVs, the answer lies in their incredible energy density.
Think of a battery as a system with two separate tanks of "energy particles" (lithium ions). Charging forces the particles from one tank to the other, storing energy. Discharging lets them flow back, releasing that energy to power your device.
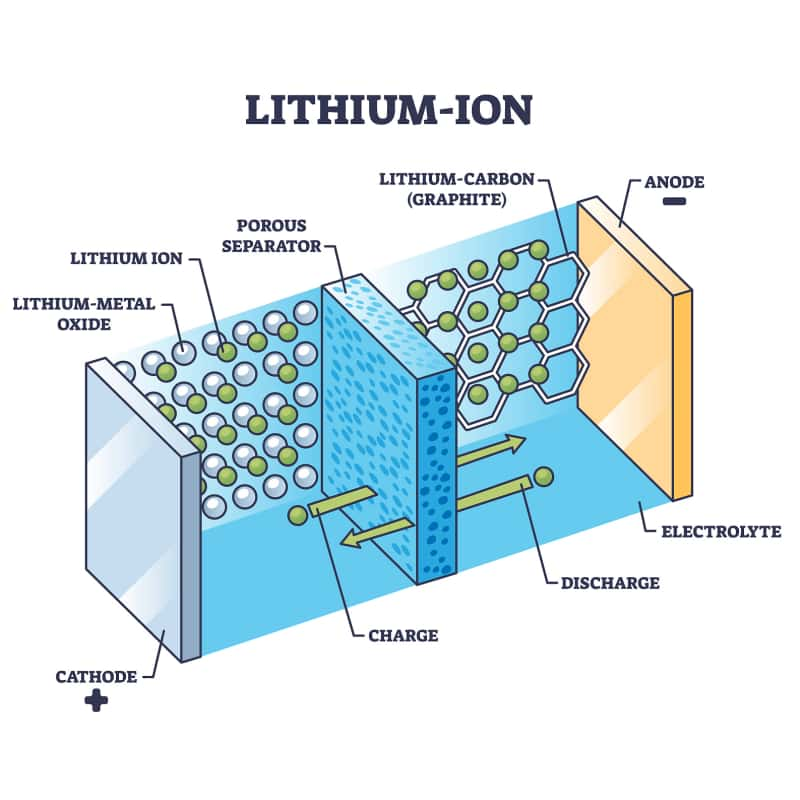
Anode (Negative Electrode): Usually made of graphite (carbon). This is the "tank" where lithium ions are stored when the battery is charged.
Cathode (Positive Electrode): Made of a lithium metal oxide (like Lithium Cobalt Oxide or Lithium Iron Phosphate). This is the other "tank."
What is the role of the electrolyte in a lithium-ion battery?: A liquid or gel that sits between the anode and cathode. It allows lithium ions to move through it, but blocks electrons (this is key!).
Separator: A porous membrane inside the electrolyte that keeps the anode and cathode from touching (which would cause a short circuit).
External Circuit: The wire or path that connects the anode and cathode outside the battery. This is where electrons flow to power your device.
When you turn on your device, you close the circuit, and a chemical reaction starts spontaneously.
Lithium Ions Travel: Lithium ions stored in the graphite anode detach and swim through the electrolyte to the cathode.
Electrons Take the Scenic Route: For every ion that moves, an electron is released from the anode. The electrolyte blocks electrons, so they are forced to take the external circuit (through your phone's components) to get to the cathode. This flow of electrons is electricity, powering your device.
Reunion: At the cathode, the electrons and lithium ions reunite and embed themselves into the cathode's material.
Discharge Summary:Anode (Graphite + Li) → Electrons (power device) + Li⁺ (through electrolyte) → Cathode (Li recombines)
Energy is released.
Plugging in applies an external electrical force that pushes the system in reverse.
External Power Pushes Electrons: The charger forces electrons to flow back from the cathode to the anode.
Lithium Ions Follow: This pulls the lithium ions out of the cathode material, forcing them to swim back through the electrolyte to the anode.
Storage: The ions re-embed themselves into the graphite structure of the anode, ready for the next discharge cycle.
Charge Summary:Cathode (Li) → Li⁺ (forced through electrolyte by charger) → Anode (Graphite + Li)
Energy is stored.
Imagine a waterwheel that powers a mill:
The Charged State: All the water (lithium ions) is in the top bucket (Anode). This represents stored energy.
Discharging: You open the gate. Water flows down from the top bucket, through the waterwheel (your device), into the bottom bucket (Cathode). The spinning waterwheel does work.
Charging: You use a pump (your charger) to force the water from the bottom bucket back up to the top bucket, storing energy again.
Leave A Message
Scan to Wechat :

Hi! Click one of our members below to chat on